Cell therapies
Cells intended for the transplantation of hematopoietic progenitors, cellular therapies, or the manufacture of advanced therapy medicinal products
The Cellular Therapy Service of the BST aims to prepare therapeutic cells for hematopoietic stem cell transplantation to treat diseases such as leukaemias or lymphomas, other cellular therapies, or the manufacture of advanced therapy medicinal products. These cells are obtained from voluntary or directed donations, processed, stored, and supplied to hospitals for administration to patients.
The Cellular Therapy Service has 3 areas:
- Cell Banks consist of lists of Donors' data (Registries) or collections of their donated cells (Banks) so that they can be selected based on the best option according to compatibility in a recipient. The STC has the Barcelona Cord Blood Bank, the REDMO registry, and the ReDoCel registry, as well as the IPS-PANIA iPSC bank.
- Clinical and Laboratory Services of Cellular Therapy: in these services, at the time of donation, the patient who will receive the cellular therapy is already known, as well as the necessary processes to prepare it. The stages include cell collection, processing, storage, distribution, and delivery.
- Research is focused on the development of new products and the improvement of transplantation and its immunological monitoring.
Cell Therapy Service
The BST cell therapy service aims to produce therapeutic cells for hematopoietic stem cell transplantation to treat diseases such as leukemia or lymphoma, other cellular therapies, or the manufacturing of advanced therapy medicinal products. These cells are obtained through voluntary or directed donations, processed, stored, and supplied to hospitals for administration to patients.
The cell therapy service has 3 areas:
- Cell banks, which consist of registries with Donor data (Registers) or collections of their donated cells (Banks) to be selected based on the best match for a recipient. The STC includes the Barcelona Cord Blood Bank, the REDMO register, the ReDoCel register, and the iPSC IPS-PANIA bank.
- Clinical services and cell therapy laboratory, where, at the time of donation, the patient who will receive the cell therapy is already identified, along with the necessary processes for its preparation. The stages include cell collection, processing, storage, distribution, and delivery.
- Research, focused on the development of new products and the improvement of transplantation and its immunological monitoring.
Cell Bank
Many patients do not have family donors available to receive a transplant or cellular therapy. At the STC, we manage the Cord Blood Bank, the donor center of the Bone Marrow Donor Registry (REDMO), and ReDoCel. Additionally, donations are used either directly as starting material to manufacture medicines or for research purposes through the BST Biobank.
The banks are authorized by the Department of Health. They hold international and national quality certifications such as NetCord-FACT and CAT, which ensure the reliability of our procedures. The REDMO donor center has registered more than 60,000 donors. The Barcelona Cord Blood Bank has 20,000 cord blood donors who have contributed to over 2,000 transplants during its 28 years of existence. The Defense Bank already has a specific donor registry (ReDoCel) and cell lines created to treat infections (T-Celbanc). The induced pluripotent stem cell (iPSC) bank IPS-PANIA has seven clinical-grade lines available as starting material for regenerative medicine immunotherapy.
CELL BANK CONTACTS
- Laura Medina, hematologist responsible for the Bone Marrow Donor Registry donor center in Catalonia.
lmedina@bst.cat - Elisenda Farssac, nurse coordinator of the interterritorial Concordia program for umbilical cord blood donation
efarssac@bst.cat - Nerea Castillo, hematologist at the Cord Blood Bank and the Defense Bank
ncflores@bst.cat - Belén Alvarez, Lead Researcher of the IPS-PANIA bank
abalvarez@bst.cat
Cord Blood Bank
The Banc de Sang i Teixits (BST) leads CONCORDIA, a cooperation program between different territories to promote and ensure the opportunity for all pregnant women to donate umbilical cord blood (UCB).
This program is based on a relationship of trust between the Banc de Sang i Teixits, the Department of Health of the Generalitat de Catalunya, and the health departments of the other participating regions: Balearic Islands, Aragon, Navarra, Extremadura, Cantabria, and the Principality of Andorra.
Within the framework of the Program, the Banc de Sang i Teixits (BST) handles cryopreservation, quality control, storage, and distribution to streamline research, improve outcomes, and ensure economic efficiency by centralizing donations in a single hub.
In 2009, CONCORDIA became the cord blood bank with the most transplants in Europe, and currently, more than 2,000 patients worldwide have found their treatment option through the Concordia program. Furthermore, since 2015, over 160 patients have received eye drops derived from cord blood hemocomponents, and the program has participated in multiple research projects to advance new therapies.
The CONCORDIA Program includes more than 40 maternity centers from 6 Spanish regions and the Principality of Andorra. It has over 20,000 high-quality UCB units stored at the BST, registered and available in national and international registries for any patient who needs them. In Spain, we rely on the REDMO registry, managed by the Josep Carreras Foundation, aimed at ensuring that a sample collected and stored in Spain can be a treatment option for any patient worldwide who requires it.
Clinical Services
The STCA is organized with a central node (cell therapy laboratory) equipped with cell processing technology (hub) and donation and application units for therapeutic cells at hospitals (spoke).
Clinical services connect the donor with the patient. At the BST center in the hospital, the donation is performed and then transported to the processing and storage node. The prepared product is returned to the BST center at the hospital for the healthcare staff to apply it together with the staff from transplant and advanced therapy units.
The entire network and the cell therapy laboratory are authorized by the Department of Health and hold quality certifications such as JACIE, which validate the entire production chain. Additionally, we support the integration of advanced therapies into clinical practice with a cryogenic drug warehouse and a drug manufacturer authorized by the Department of Health and the Spanish Agency for Medicines and Medical Devices (AEMPS).
The cell therapy laboratory supports most transplant programs in Catalonia. It enables conventional cell processing: autologous and allogeneic (related, unrelated, haploidentical, and cord blood). It also includes special processes such as plasma washing in apheresis, volume and red blood cell depletion in bone marrow, and positive selections (CD34+ and others).
Through apheresis and processing when necessary, the STC enables treatment with clinical CARTs and participation in industry clinical trials, with more than 100 CARTs per year.
Cord multicomponents generate two products for regenerative medicine: eye drops and gels derived from cord blood.
- Eye Drops (CBED) is a drug derived from cord platelet lysate, rich in growth factors, obtained from platelet concentrate (800-1200x10^9/L). A batch contains 19 frozen vials (-80º C).
- Gel (CBPG) is a drug derived from platelet concentrate (800-1200x10^9/L). It is stored frozen (-80º C).
CONTACTS FOR CLINICAL SERVICES AND LABORATORIES
- Jesús Fernández, director and medical director of clinical services.
jefernandez@bst.cat - David Gómez, nurse coordinator of the STCA for cellular therapies.
dgomez@bst.cat - Carmen Azqueta, technical director of the cell therapy laboratory.
cazqueta@bst.cat - Dinara Samarkanova, medical specialist in cord multicomponent-derived products.
dsamarkanova@bst.cat -
For patient management, contact cbed@bst.cat
Research
Within the STC research area, there are different lines of research grouped as follows:
- TACT: Umbilical cord blood (UCB): clinical transplant studies using umbilical cord blood source, mobilized/non-mobilized apheresis, cell manipulation: retrospective clinical studies with clinical and biological databases to improve transplantation.
- CASH: Development of personalized hemotherapy from UCB multicomponents.
- iPDA: Development of cellular immunotherapy from the pluripotent stem cell bank.
- BACT: Research in Bioprocesses and Regenerative Medicine (BACT).
- AICT: Immunotherapy Research: developing a research line on "Antiviral T Lymphocytes".
RESEARCH CONTACTS
-
Jesús Fernández and Nerea Castillo, TACT
jefernandez@bst.cat, ncastillo@bst.cat -
Dinara Samarkanova, CASH
dsamarkanova@bst.cat -
Belén Álvarez-Palomo, iPDA
abalvarez@bst.cat -
Joaquim Vives, BACT
jvives@bst.cat -
Francesc Rudilla, AICT
BACT in 2024:
-
Dr. Joaquim VIVES (group leader)
-
Dr. Raquel CABRERA-PÉREZ (postdoc)
-
Dr. Sara MORINI (postdoc)
-
Carmen PASTOR QUIÑONES
-
Sara MARQUINA, TFM internship
-
Teresa RIOS LAABOUDI, TFM internship
-
Noèlia MUÑOZ, TFG internship
-
Irene PORTAS TORRES, predoctoral collaboration VHIR
-
Víctor GARCIA-GRAGERA, predoctoral collaboration IQS
-
Sílvia TORRENTS (part-time predoctoral)
-
Dr. Margarita CODINACH (part-time)
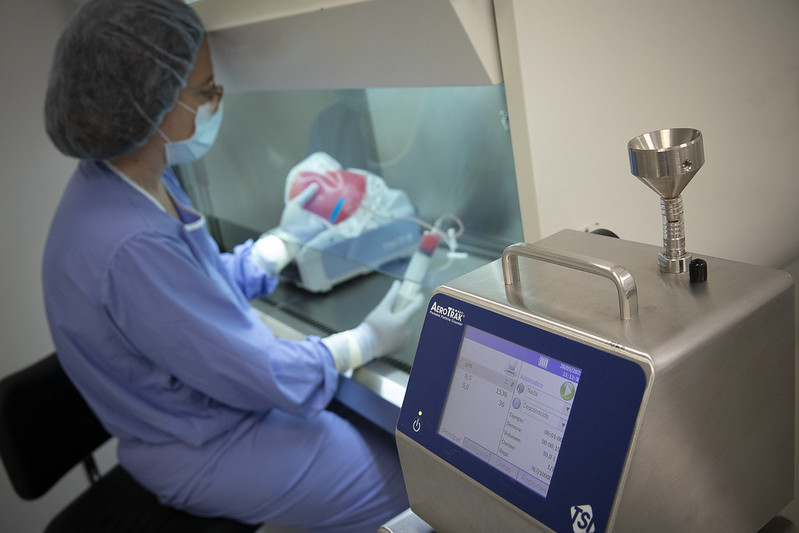
Facilities, equipment, and professionals
The facilities dedicated to cell therapies occupy a space of approximately 40.7 m2 (room number 5) and a multicomponent room of 28.9 m2.
Both spaces are located on the -1 floor of the Dr. Frederic Duran i Jordà Building in Barcelona and have D-grade qualification:
-
-1 floor with 2 D-class rooms assigned to the cell therapy laboratory, equipped with laminar flow hoods, automated processors, centrifuges, and sterile connectors.


We also have facilities dedicated to cryopreservation activities.
-
Cryopreservation (-1 floor) with 7 bioarchive tanks dedicated to UCB, 17 nitrogen tanks (2 of them specific for medication storage), and 11 freezers at -80ºC.
The cell therapy service includes 2 hematologists, 1 technical director, 2 process specialists, 9 laboratory technicians, 1 Concordia program coordinator, 1 DUI coordinator, 3 administrative staff, 1 data manager, and 1 laboratory assistant. Functionally, it is connected to 9 BST centers with a hematologist, DUI coordinator, and 3 researchers.
Healthcare professional, you need to place an order

To place orders and requests, you need to access the tool within the Private Area. You can access it from here or, if you have any questions or queries, please contact us.
subministrament@bst.catLatest news
Advanced Therapies

Advanced therapies are characterized by using genes, tissues, or human cells that are manipulated to turn them into a medicine.






