Advanced Therapies
They are innovative therapies, based on knowledge and state-of-the-art techniques, that offer great hope for improving the treatment of diseases that have been incurable and difficult to tackle.
Development of Advanced Therapies
Advanced Therapy Medicinal Products (ATMPs) represent a leading category of innovative medical treatments based on gene modification (gene therapy), cells (cell therapy), or tissues (tissue engineering).
These therapies are developed and manufactured under strict Good Manufacturing Practices (GMP) standards to ensure their safety, quality, and efficacy for patient use.
At the Blood and Tissue Bank (BST), we have experience in the production of advanced cellular therapies such as CAR-T cells, TILs (Tumor-infiltrated lymphocytes), mesenchymal stem cells (hMSC), exosomes, various viral vectors (retrovirus, lentivirus, etc.), or specific T-viruses, providing new treatment options for patients.
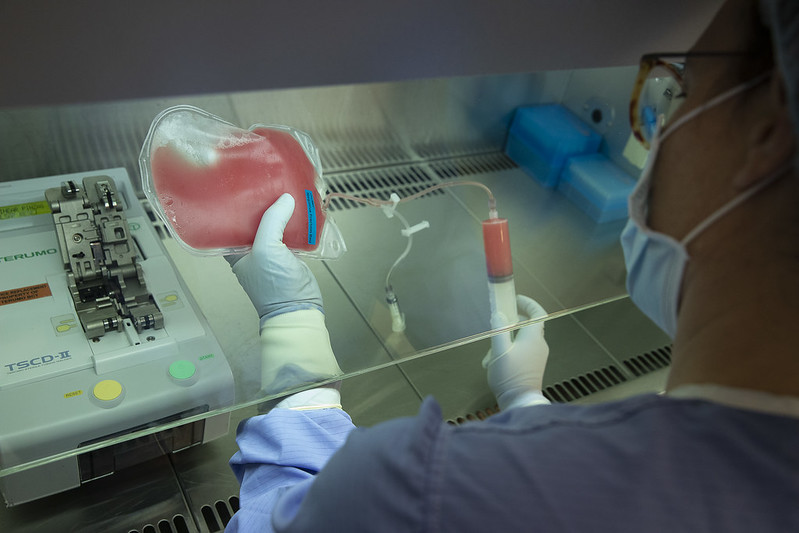
Therapies
Advanced therapies are human-use medicines based on genes (gene therapy), cells (cell therapy), or tissues (tissue engineering). These are innovative therapies based on cutting-edge knowledge and techniques that offer great hope for improving the treatment of previously incurable and difficult-to-treat diseases.
These therapies or processes go beyond conventional methods and focus on using cells, tissues, or genes to restore, improve, or replace damaged or diseased functions of the body.
Therapies in development
- CAR-T: CAR-T therapy uses genetically modified T cells from the patient to specifically and effectively identify and destroy cancer cells. This innovative immunotherapy has shown great success in certain types of cancer, such as leukemias and lymphomas.
- Exosomes: Exosomes are small extracellular vesicles that transport molecules such as proteins and RNA between cells, playing a key role in cell communication and the regulation of biological processes. They are being studied for their potential use in regenerative therapies and in the treatment of various diseases.
- iPSC: Induced pluripotent stem cells (iPSC) are adult cells genetically reprogrammed to return to a pluripotent state, similar to that of embryonic stem cells, capable of differentiating into any cell type in the body. They are a promising tool for biomedical research and cell therapies.
- Expanded Virus-specific T-cells (VST): Virus-specific T cells (VST) are immune cells designed to recognize and destroy cells infected by specific viruses, offering a targeted treatment for persistent and resistant viral infections.
Therapy production
- TILs: Tumor-infiltrating lymphocytes (TILs) are immune cells extracted from the patient’s tumor, cultured and activated in the laboratory, and reintroduced into the patient to fight cancer from within the tumor.
- Lentiviral and Retroviral Vectors: Viral vectors, such as lentiviruses and retroviruses, are molecular tools used to introduce genetic material into cells for therapeutic or research purposes. These vectors enable stable genetic modification of cells to treat various genetic diseases and cancers.
-
CD133+ Cell Therapy: CD133-based therapies use hematopoietic stem cells and endothelial progenitors identified by the expression of the CD133 marker to promote tissue regeneration and treat various diseases.
- hMSC: Human mesenchymal stem cells (hMSC) are multipotent cells that can differentiate into various cell types, such as osteocytes, chondrocytes, and adipocytes. They are used in regenerative therapies to repair and regenerate damaged tissues, as well as in the allogeneic treatment of graft-versus-host disease (GVHD).
- Virus-specific T-cells (VST): Virus-specific T cells (VST) are immune cells designed to recognize and destroy cells infected by specific viruses, offering a targeted treatment for persistent and resistant viral infections.
Services
- Design and scaling of manufacturing processes according to GMP (Good Manufacturing Practice) standards: We design and scale manufacturing processes according to GMP standards, ensuring the production of high-quality and safe Advanced Therapy Medicinal Products (ATMPs).
- Process development: Our process development services optimize and validate the methods used to produce ATMPs, improving efficiency and ensuring compliance with regulatory standards.
- Storage facilities for distribution to healthcare centers: We offer state-of-the-art storage facilities for the distribution of ATMPs to healthcare centers, maintaining optimal conditions to preserve product integrity.
- Cryopreservation and/or cryoconservation of ATMPs: Our cryopreservation and cryoconservation services ensure the long-term preservation of ATMPs, maintaining their viability and functionality for future use.
- Stability studies: We evaluate the viability and safety of ATMPs over time, under both cryopreservation and storage conditions. Our studies ensure their quality until the moment of administration.
- Logistics for the shipment of ATMPs under controlled conditions to healthcare centers: We provide comprehensive logistical services for the shipment of ATMPs under controlled conditions to healthcare centers, ensuring safe and timely delivery.
- Regulatory services: Our regulatory services support the compliance and approval process of ATMPs, assisting with documentation, submissions, and interactions with regulatory authorities.
- Quality control services: We provide rigorous quality control services to ensure that all ATMPs meet the highest standards of safety, efficacy, and quality before reaching patients.
Facilities
The BST has 9 cleanrooms distributed across two floors, occupying approximately 200m2.
These cleanrooms are approved by the AEMPS for conducting Advanced Therapy Medicinal Product (ATMP) production activities in a Good Manufacturing Practice (GMP) environment.
Currently, there are 5 grade B rooms, 1 grade C room, and 3 grade D rooms.
The manufacturing activities that can be performed with the current infrastructure include:
- 10 workstations in 4 cleanrooms for open system manufacturing (e.g., G-Rex or Cell-stack cell expansion platforms).
- 2 production stations for automated cell separation and transfection in closed systems (e.g., CliniMACs Prodigy), distributed across 2 separate cleanrooms.
- 1 area for the production of genetically modified organisms (lentiviral vectors), distributed across 2 separate cleanrooms.
- 2 support areas for final product conditioning and bioreactors, distributed across 2 separate cleanrooms.



Contact
- Cristina Prat, Technical Director
cprat@bst.cat - Marga Codinach, Head of Quality Control
mcodinach@bst.cat - Patricia Sagre, Head of Quality Assurance
psagre@bst.cat
The BST is a member of the Advanced and Emerging Therapies Network of Catalonia.
The Blood and Tissue Bank (BST) is part of the Advanced and Emerging Therapies Network of Catalonia, coordinated by Biocat, which promotes research and development to make these therapies more accessible to the public.
