Environmental microbiological control of surfaces
Code: 60000, 60006
Type: Control de qualitat de medicaments de teràpia avançada
Technical information
Utility
Clean rooms or white rooms are specially designed to ensure low levels of contamination and have environmental parameters strictly controlled by regulations. Environmental microbiological controls allow us to know the microbiological conditions of the facilities, equipment, and operators, in order to ensure compliance with regulations.
The most common origin of microorganisms found in a facility is:
-
The personnel entering the facility.
-
The materials entering the facility.
-
The air filtration system (HEPA filters for clean rooms).
The elements that can become contaminated with microorganisms and pose a risk are:
-
Air: Environmental air or laminar flow hoods
-
Surfaces: Floors, walls, tables, handles, etc.
-
Equipment: Incubators, laminar flow hoods, centrifuges, refrigerators, microscopes, balances, etc.
-
Clothing: Gloves, operator's clothing.
The definition of environmental control points and frequencies is based on an analysis of environmental risk conducted before the start of activities in a facility, which includes reviewing the rooms, equipment, materials, workflows, the number of people working there, and other factors.
Method
The plates used for environmental microbiological control are shown in Table 1. The plates contain suitable culture media for the growth of bacteria and fungi. They also include agents that neutralise the possible presence of disinfectants to prevent interference with the growth of microorganisms.
Before using a batch of plates, a growth promotion test is performed to verify if that batch of plates allows the growth of microorganisms.
|
Medium |
Technique |
Element to control |
Microorganisms |
|
TSA contact with neutralising agents 55 mm plate |
Contact |
Surfaces |
Bacteria |
|
SDA contact with neutralising agents 55 mm plate |
Fungi |
||
|
TSA with neutralising agents 90 mm plate |
Volumetric air |
Air |
Bacteria |
|
SDA with neutralising agents 90 mm plate |
Fungi |
||
|
TSA with neutralising agents 90 mm plate |
Sedimentation air |
Air |
Bacteria |
|
SDA with neutralising agents 90 mm plate |
Fungi |
||
|
TSA with neutralising agents 90 mm plate |
Fingerprints |
Gloves |
Bacteria |
|
SDA with neutralising agents 90 mm plate |
Fungi |
Table 1. TSA: Tryptone Soya Agar medium. SDA: Sabouraud Dextrose Agar medium
The most suitable environmental microbiological control techniques for the points to be monitored are shown in Table 2:
|
Element |
Technique |
|
Air |
Volumetric or sedimentation air sampling |
|
Surfaces |
Contact |
|
Operator |
Glove prints |
Table 2: Techniques used to sample different elements.
The contact sampling technique involves placing the plate in contact with the surface to be studied, as seen in Figure 1. Two plates are used, one for fungi and one for bacteria. It is important to follow the manufacturer's instructions for the plates. An applicator can be used to ensure that the plate's contact time and pressure on the surface are correct. If done manually, ensure that the entire plate is evenly in contact with the surface for the specified time and pressure as indicated by the manufacturer.
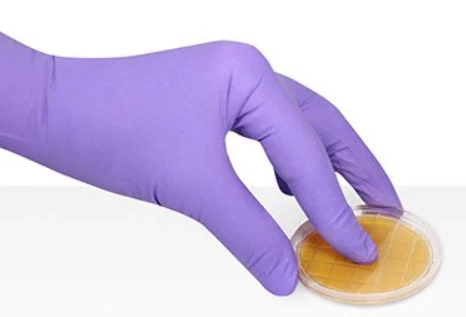
Figure 1: Contact sampling
The plates should be sent to the Microbiology Laboratory as soon as possible after sampling. There, they are received and incubated:
-
TSA plates are incubated at 32.5°C (30°C - 35°C) for 3-5 days for bacteria
-
SDA plates are incubated at 22.5°C (20°C - 25°C) for 5-7 days for fungi.
Specimen information
Sample: Surfaces (contact)
Tubes: 55 mm TSA plate for bacteria and SDA for fungi
Stability: Transport immediately to the laboratory. If not possible, store at room temperature for a maximum of 4 hours.
Transport instructions: Room temperature.
Reason for rejection: Plate not closed properly, unidentified, etc.
Administrative information
BST Code: 60000 (bacteria) and 60006 (fungi)
Old BST Code: 7534 (bacteria) and 7553 (fungi)
Test Description: Surface bacteria control (contact), Surface fungi control (contact)
Synonyms: Environmental microbiological control of surfaces (contact), Environmental Microbiological Control of Surfaces (contact)
Section: Microbiology
BST Rate: Check the updated rates here.
Profiles
Not applicable
References
-
Guide to the quality and safety of tissues and cells for human application. 5th edition EDQM, 2022
-
Farmacopea Europea 11ena edició, 2023
-
ISO 171330-1:2008 Calidad ambiental en interiores. Parte 1: Diagnóstico de calidad ambiental interior.
-
ISO 171330-2:2014 Calidad ambiental en interiores. Parte 2: Procedimientos de inspección de calidad ambiental interior.
-
ISO 171330-3:2014 Calidad ambiental en interiores. Parte 3: Sistema de gestión de los ambientes interiores.
-
ISO 171340:2020 Validación y cualificación de salas de ambiente controlado en hospitals
-
Norma EN ISO 14644-1. Clasificación de sala limpias.Estándares en hemoteràpia CAT
-
FACT-JACIE International Standards for Cellular Therapy Product Collection, Processing, and Administration.
-
NETCORD-FACT International Standards for Cord Blood Collection, Banking, and Release for Administration.
-
NCF: Guía de Normas de Correcta Fabricación de Medicamentos de Uso Humano y Veterinario. Anexo 1
-
NCF Parte IV: Directrices sobre normas de correcta fabricación específicas para Medicamentos de Terapia Avanzada.
-
GpG. Directrices de Buenas Prácticas - Guía para la preparación, uso y control de calidad de los componentes sanguíneos.
-
Real Decreto-ley 9/2014, de 4 de julio, por el que se establecen las normas de calidad y seguridad para la donación, la obtención, la evaluación, el procesamiento, la preservación, el almacenamiento y la distribución de células y tejidos humanos y se aprueban las normas de coordinación y funcionamiento para su uso en humanos.